Contents
NEET Chemistry Chapter Wise Mock Test – p-Block Elements
Question 1.
Which of the following compound is tribasic acid?
(a) H3PO2
(b) H3PO3
(c) H3PO4
(d) H4P2O7
Question 2.
Among the members of VA group (N, P, As, Sb and Bi), which of the following properties shows an increase as we go down from nitrogen to bismuth?
(a) lectronegativity
(b) Acidic nature of the pentoxide
(c) Stability of -3 oxidation state
(d) Reducing character of hydrides
Question 3.
Which is the most thermodynamically stable allotropic form of phosphorus?
(a) Red
(b) Yellow
(c) White
(d) Black
Question 4.
Which of the following is not known?
(a) NI3
(b) NCl3
(c) NCl5
(d) SbCl3
Question 5.
The following are some statements related to VA group hydrides,
I. Reducing property increases from NH3 to BiH3.
II. Tendency to donate lone pair decreases from NH3 to BiH3.
III. Thermal stability of hydrides decreases from NH3 to BiH3.
IV. Bond angle of hydrides decreases form NH3 to BiH3.
The correct statements are
(a) I, II, III and IV
(b) I, III and IV
(c) I, II and IV
(d) I and IV
Question 6.
A black compound of manganese reacts with a halogen acid to give greenish yellow gas. When excess of this gas reacts with NH3 an unstable trihalide is formed. in this process the oxidation state of nitrogen changes from
(a) -3 to +3
(b) -3 to 0
(c) -3 to +5
(d) 0 to -3
Question 7.
Elements of group -15 form compounds in +5 oxidation state. However, bismuth forms only one well characterised compound in +5 oxidation state. The compound is
(a) Bi2O5
(b) BiF5
(c) BiCl5
(d)Bi2S5
Question 8.
As the number of —OH groups increases in hypophosphorous acid, orthophosphorus acid and orthophosphoric acid, the acidic strength
(a) increases
(b) decreases
(c) remains nearly same
(d) remains appropriately same
Question 9.
Which of the following is the most explosive?
(a) NCl3
(b) PCl3
(c) AsCl3
(d) All of these
Question 10.
In qualitative analysis, when H2S is passed through an aqueous solution of salt acidified with dil. HCl, a black precipitate is obtained. On boiling the precipitate with dil. HNO3, it forms a solution of blue colour. Addition of excess of aqueous solution of ammonia to this solution gives
(a) deep blue precipitate of Cu(OH)2
(b) deep blue solution of [Cu(NH3)4]2+
(c) deep blue solution of Cu (NO3)2
(d) deep blue solution of Cu(OH)2 Cu(NO3)2
Question 11.
Sodium pyrophosphate is represented by which of the following formula?
(a) Na2 P2O4
(b) Na4P2O5
(c) Na4P2O7
(d) Na2P2O5
Question 12.
In solid state,PCl5is a
(a) covalent solid
(b) octahedral structure
(c) ionic solid with [PCl6]+ octahedral and [PCI4]+ tetrahedral
(d) ionic solid with [PCl4]+ tetrahedral and [PCl6]– octahedral
Question 13.
On heating lead (II) nitrate gives a brown gas A. The gas A on cooling changes to colourless solid B. Solid B on heating with NO changes to a blue solid C. Identify C.
(a) NO2
(b) N2O4
(c) N2O5
(d) N2O3
Question 14.
On heating compound (A) gives a gas (B) which is a constituent of air. This gas when treated with 3 moles of hydrogen (H2) in the presence of a catalyst gives another gas (C) which is basic in nature. Gas (C) on further oxidation in moist condition gives a compound (D) which is a part of acid rain. Identify compound (D).
(a) HNO2
(b) HNO3
(c) H2SO4
(d) HCl
Question 15.
The substance used in Holme’s signals of the ship is a mixture of
(a) CaC2 + Ca3P2
(b) Ca3(PO4)2 + Pb3O4
(c) H3PO4 + CaCl2
(d) NH3 + HOCl
Question 16.
Which of the following are peroxoacids of sulphur?
(a) H2SO5 and H2S2O8
(b) H2SO5 and H2S2O7
(c) H2S2O7 and H2S2O8
(d) H2S2O6 and H2S2O7
Question 17.
Which of the following salt would give SO2 with hot and dil. H2SO4 and also decolourises Br2 water?
(a) Na2SO3
(b) NaHSO4
(c) Na2SO4
(d) Na2S
Question 18.
Which one of the following oxides is expected to exhibit paramagnetic behaviour?
(a) CO2
(b) SO2
(c) ClO2
(d) SiO2
Question 19.
Which of the following attacks glass?
(a) HCl
(b) HF
(c) HI
(d) HBr
Question 20.
F2 formed by reacting K2MnF6 with
(a) SbF5
(b) MnF3
(c) KSbF6
(d) MnF4
Question 21.
In the manufacture of bromine from sea water, the mother liquor containing bromides is treated with
(a) I2
(b) Cl2
(c) CO2
(d) SO2
Question 22.
Which of the following statement is not true?
(a) HF is stronger acid than HCl
(b) Among halide ions, iodide is the most powerful reducing agent
(c) Fluorine is the only halogen that does not show a variable oxidation state
(d) HOCl is a stronger acid than HOBr
Question 23.
The correct order of acidic strength is
(a) CO2 > N2O5 > SO3
(b) Cl2O7 > SO2 > P4O10
(c) Na2O > MgO > Al2O3
(d) K2 > CaO > MgO
Question 24.
Which one of the following is a pseudo halide?
(a) I3–
(b) IF–
(c) ICl
(d) CN–
Question 25.
What is the oxidising agent in chlorine water?
(a) HCl
(b) HClO2
(c) HOCl
(d) None of these
Question 26.
Which of the following halides is least stable and has doubtful existence?
(a) Cl4
(b) SnI4
(c) PbI4
(d) GeI4
Question 27.
Reduction potentials of some ions are given below.
Arrange them in decreasing order of oxidising power.

Question 28.
In Fischer Ringe’s method of separation of noble gas mixture from air is used
(a) 90% CaC2 + 10%CaCl2
(b) coconut charcoal
(c) sodalime + potash solution
(d) 90% CaCO3 + 10% urea
Question 29.
The charcoal maintained at 100°C absorbs
(a) Ne and Kr
(b) Ar, Kr, Xe
(c) He and Ar
(d) He and Ne
Question 30.
In Ramsay and Rayleigh’s isolation of noble gases from air, the nitrogen of the air is finally converted into
(a) Only NaNO2
(b) NO and NO2
(c) Only NaNO3
(d) NaNO2 and NaNO3
Question 31.
Xenon hexafluoride reacts with silica to form a xenon compound X. The oxidation state of xenon in Xe is
(a) +2
(b) +4
(c) +6
(d) 0
Question 32.
Which of the following is not obtained by direct reaction of constituent elements?
(a) XeO3
(b) XeF2
(c) XeF6
(d) XeF4
Question 33.
Which of the following gas mixture is used by the divers inside the sea?
(a) O2 + He
(b) O2 + Xe
(c) O2 + Ar
(d) O2 + N2
Question 34.
Which one of the following reaction of xenon compounds is not feasible?
(a) XeO3 + 6HF ——-> XeF6 + 3H2O
(b) 3XeF4 + 6H2O ———-> 2Xe + XeO3 + 12HF + 1.5O2
(c) 2XeF2 + 2H2O ———> 2Xe + 4HF + O2
(d) XeF6 + RbF —–> Rb[XeF7]
Question 35.
Which among the following statements are correct?
I. Carbon monoxide is neutral where as SO3 is acidic.
II. Potassium oxide is basic whereas nitrous oxide is acidic.
III. Aluminium and zinc oxides are amphoteric.
IV. Sulphur trioxide is acidic whereas phosphorus pentoxide is basic.
(a) (II) and (V)
(b) (I) and (IV)
(c) (I) and (III)
(d) (II) and (IV)
Question 36.

Question 37.
Noble gases are sparingly soluble in water due to
(a) dipole-dipole interaction
(b) dipole-induced dipole interaction
(c) induced dipole-induced dipole interaction
(d) hydrogen bonding
Direction (Q.Nos.38-39): In the following questions more than one of the answers given may be correct. Select the correct answer and mark it according to the codes.
Codes
(a) 1,2 and 3 are correct
(b) 1 and 2 are correct
(c) 2 and 4 are correct
(d) 1 and 3 are correct
Question 38.
White phosphorus has
1. six P—P single bonds
2. four lone pairs of electrons
3. P—P—P angle of 60°
4. four P—P single bonds
Question 39.
Which of the following can act as a Lewis base?
1. NCl3
2. PCl3
3. NBr3
4. SbCl3
Question 40.

Question 41.

Direction (Q. Nos. 42-47): Each of these questions contains two statements : Assertion and Reason. Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
(a) Assertion is true, Reason is true; Reason is the correct explanation for Assertion
(b) Assertion is true, Reason is true; Reason is not the correct explanation for Assertion
(c) Assertion is true, Reason is false
(d) Assertion is false, Reason is true
Question 42.
Assertion: Among chalcogens, tendency of catenation is maximum for sulphur.
Reason: S—S bond dissociation energy is higher than O—O bond dissociation energy.
Question 43.
Assertion: White phosphorus is stored under water.
Reason: White phosphorus is highly reactive and catches fire spontaneously in air.
Question 44.
Assertion: F—F bond in F2 molecule is strong.
Reason: F-atom is small in size.
Question 45.
Assertion: OF2 is named as oxygen difluoride.
Reason: In OF2, oxygen is less electronegative than fluorine.
Question 46.
Assertion: F2 has high reactivity.
Reason: F—F bond has low bond dissociation enthalpy.
Question 47.
Assertion: Noble gases have positive electron affinity.
Reason: Noble gases have stable closed shell electronic configuration
Question 48.
Which is the strongest acid in the following?
(a) HClO3
(b) HClO4
(c) H2SO3
(d) H2SO4
Question 49.
Which of the following does not give oxygen on heating?
(a) Zn(ClO3)2
(b) K2Cr2O7
(c) (NH4)2Cr2O7
(d) KCl03
Question 50.
Which of the following is polar molecule?
(a) SF4
(b) SiF4
(c) XeF4
(d) BF3
Question 51.
XeF2 is isostructural with
(a) ICl2
(b) SbCl3
(c) BaCl2
(d) TeF2
Question 52.
Which of the following statements is not valid for oxoacids of phosphorus?
(a) Orthophosphoric acid is used in the manufacture of triple superphosphate
(b) Hypophosphorous acid is a diprotic acid
(c) All oxoacids contain tetrahedral four coordinated phosphorus
(d) All oxoacids contain at least oneP = O unit and one P—OH group
Question 53.
Sulphur trioxide can be obtained by which of the following reaction?
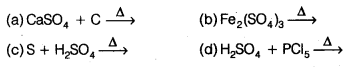
Question 54.
Which of the following dissolves in water but does not give any oxyacid solution?
(a) SO2
(b) OF2
(c) SCl4
(d) SO3
Question 55.
Correct order of decreasing thermal stability is as
(a) NH3 > PH3 > AsH3 > SbH3
(b) PH3 > NH3 > AsH3 > SbH3
(c) AsH3 > PH3 > NH3 > SbH3
(d) SbH3 > AsH3 > PH3> NH3
Question 56.
The number of S—S bonds in sulphur trioxide trimer (S3O9) is
(a) 0
(b) 1
(c) 2
(d) 3
Question 57.
Which one of the following is present as an active powder for bleaching action?
(a) Ca(OCl)2
(b) CaO2Cl
(c) CaCl2
(d) CaOCl2
Question 58.
When cone. H2SO4 is heated with P2O5, the acid is coaverted into
(a) sulphur trioxide
(b) sulphur dioxide
(c) sulphur
(d) a mixture of sulphur dioxide and sulphur trioxide
Question 59.
Which of the following is not a peroxy acid?
(a) Perphosphoric acid
(b) Pernitric acid
(c) Perdisulphuric acid
(d) Perchloric acid
Question 60.
The tendency of BF3, BCl3 and BBr3 to behave as Lewis acid decreases in the sequence
(a) BCl3 > BF3 > BBr3
(b) BBr3 > BCl3 > BF3
(c) BBr3 > BF3 > BCl3
(d) BF3 > BCl3 > BBr3
Question 61.
Bromine water reacts with SO2 to form
(a) HBr and S
(b) H2O and HBr
(c) S and H2O
(d) H2SO4 and HBr
Question 62.
Ammonia, on reaction with excess of chlorine, gives
(a) NCl3 and HCl
(b) N4 and NH4Cl
(c) NCl3 and NH4Cl
(d) N2 and HCl
Question 63.
Consider the sequence of oxides : Na2O, SiO2, P4O10.
Which factor decreases from Na2O to SiO2 and also from SiO2 to P4O10?
(a) Covalent character
(b) Melting point
(c) pH when mixed with water
(d) Solubility in aqueous alkali
Question 64.
The type of hybrid orbitals used by iodine atom in hypoiodous acid molecule are
(a) sp3
(b) sp2
(c) sp
(d) sp3d
Answers:

Hints And Solutions: