NEET Chemistry Notes Chemical Kinetics – Order of Reaction
Order of Reaction
Order of Reaction
The sum of the coefficients (or powers) of the reacting species that are involved in the rate law expression for the reaction represents the order of the reaction

The order of reaction can only be determined by experiments.
Zero Order Reaction
Reactions in which the concentration of reactants do not change with time and the concentration rates remain constant throughout are said to be zero order reactions.

Units of rate constant and rate of reaction are same, i.e. mol-1 L-1 s-1.
First Order Reaction
The first order reaction is defined as “the reaction in which the reaction rate is determined by the change of one concentration term of the reactant only”.
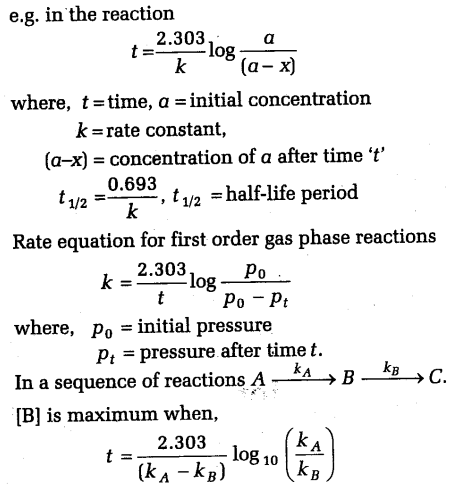
Second Order Reaction
The reaction is said to be of second order if its reaction rate is determined by the variation of two concentration terms of reactants. It can be represented as

Third Order Reaction
A reaction is said to be of third order if its rate is determined by the variation of three concentration terms.

The rate constant, k for the reaction is given by

The unit of rate constant for third order reaction is is L2 mol-2 s-1.
Reactions of third and higher orders are rare, but some examples of 3rd order reactions are definitely seen. This is due to the fact that the chances of three molecules of coming to a single point simultaneously, i.e. probability of trimolecular collisions is much less as compared to unimolecular or bimolecular collisions.
Examples of third order reactions are

Negative Order Reactions
Sometimes, the rate of reaction decreases as the concentration of one of the constituent is increased, e.g. transformation of ozone into oxygen,

Pseudo Unimolccular Reaction
When one of the reactants is present in large excess, the second order reaction confirms to the first order and is called pseudo unimolecular reaction.
e.g. hydrolysis of ester in acidic medium CH3COOC2H5
![]()
This is also known as pseudo first order reaction. Rate =k [Ester]
Molecularity Always whole number, it can never be zero, negative or fraction.
Order of reaction It may be whole number, fraction, zero or even negative.
