The study of cellular Biology Topics is essential to understanding the workings of all living organisms.
What is the Nitrogen Cycle and Why is it Key to Life?
Nitrogen is another important chemical on Earth and is present in all living organisms, in the form of protein, amino acids, and nucleic acids. It exists in the molecular form (N2) and in the form of some oxides in the atmosphere. But aerial nitrogen, the most abundant component of air (i.e., 78 percent) is chemically inert and cannot be used in its pure form by most organisms. First, it needs to be converted into nitrates (NO3) for the use of plants.

The conversion can be done either by industrial nitrogen fixation (i.e., manufacturing of ammonium salts and urea or chemical fertilizers) or by some nitrogen-fixing bacteria such as Azotobactor (occurs freely in soil) and Rhizobium (occurs in root nodules of leguminous plants as pea, gram, bean, etc.) which convert the atmospheric nitrogen into water-soluble nitrates. The process of biofixation of nitrogen is called nitrogen fixation. During lightning, nitrogen in the atmosphere reacts with oxygen to form dilute nitric acid. This acid comes down to Earth with rainwater. Nitrates are absorbed by plants into their system and Utilized for making organic matter (proteins), etc.
When animals consume plant matter, they break down the plant’s nitrogenous compounds and use them to form new animal proteins and other cell components. After an animal excretes urea or uric acid or after an animal or plant dies, certain bacteria carry out ammonification: they produce ammonium ions (NH4+) from nitrogen-containing molecules. Plants can then assimilate this ammonium ion themselves or other bacteria can change it to nitrate (NO3-) by nitrification. Plants take in some of the nitrates produced in this way.
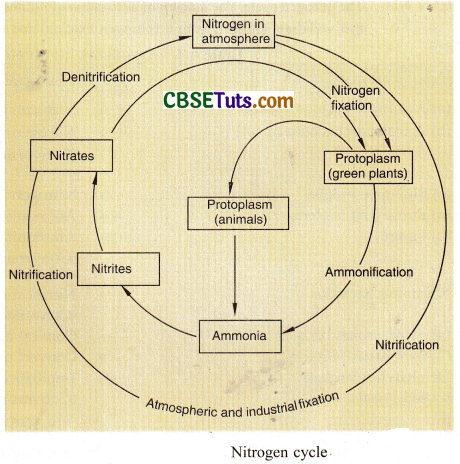
The process of ammonia formation is called ammonification. Some microorganisms (Nitrosomonas, Nitrobacter) convert ammonia into nitrates. The process is called nitrification. There are some other bacteria (decomposers, e.g., Pseudomonas) that reduce nitrates back to nitrogen or to ammonia or to some other oxides. This process is called denitrification. Free nitrogen returns to the atmospheric pool and oxides are taken up by plants.

The nitrogen cycle. The amount of nitrogen in the atmosphere is maintained by a balance between the processes that withdraw nitrogen from it (nitrogen fixation) and those which add nitrogen to it (denitrification).
Organisms involved in Nitrogen Fixation
| Name of Microorganism | Role Played in Nitrogen Cycle |
| 1(a) Rhizobium bacteria (in root nodules) | Nitrogen fixation |
| (b) Azotobactor bacteria in soil | Nitrogen fixation (Conversion of atmospheric nitrogen gas into nitrogen compounds) |
| 1(c) Blue-green Algae | Nitrogen fixation |
| 2(a) Putrefying bacteria | Ammonification |
| (b) Fungi | Ammonification (Conversion of nitrogen-containing proteins of dead plants and animals into ammonia) |
| 3. Nitrifying bacteria | Nitrification (Conversion of ammonia into nitrites and then into nitrates) |
| (a) Nitrosomonas bacteria | Convert ammonia into nitrites (NO2) |
| (b) Nitrobacter bacteria | Convert nitrites into nitrates (NO3) |
| 4. Denitrifying bacteria (called Pseudomonas) | Denitrification (Conversion of nitrate salts into free nitrogen gas) |
The nitrogen cycle is called a perfect cycle in the biosphere because it maintains the overall amount of nitrogen constant in the atmosphere, soil, and water.
The nitrogen cycle depends upon at least four different kinds of bacteria known as the decay causes, the nitrifiers, the denitrifiers, and the nitrogen fixers. There is a regular circulation of nitrogen through the air, soil, plants, and animals.
The reduction of atmospheric nitrogen (N2) to the ammonium ions (NH4+) is called nitrogen fixation. The key to biofixation is the enzyme nitrogenase which catalyzes the splitting of N2.
The nitrogen-fixing bacterium of root nodules, Rhizobium leguminosarum, is an aerobic bacterium that needs some oxygen for survival. To help it this way, leguminous plants’ root nodules have a haemoglobin-like protein called leg haemoglobin.
16 molecules of ATP are needed by the bacterium for each molecule of nitrogen that is fixed.
In the soil microorganism, Klebsiella pneumoniae a total of 17 genes, called nif genes are known to be responsible for nitrogen fixation.
Researchers in biotechnology are now attempting the transfer of ‘nif’ genes from prokaryotes to crop plants so that yield of crops such as rice and wheat may be increased.