Contents
NCERT Solutions for Class 11 Chemistry Chapter 11 The pBlock Elements includes all the important topics with detailed explanation that aims to help students to understand the concepts better. Students who are preparing for their Class 11 exams must go through NCERT Solutions for Class 11 Chemistry Chapter 11 The pBlock Elements. Going through the solutions provided on this page will help you to know how to approach and solve the problems.
Students can also find NCERT intext, exercises and back of chapter questions. Also working on Class 11 Chemistry Chapter 11 The pBlock Elements NCERT Solutions will be most helpful to the students to solve their Homeworks and Assignments on time. Students can also download NCERT Solutions for Class 11 Chemistry Chapter 11 The pBlock Elements PDF to access them even in offline mode.
Class 11 Chemistry Chapter 11 The p Block Elements NCERT Solutions
The p Block Elements NCERT Solutions
NCERT Solutions for Class 11 Chemistry Chapter 11 The pBlock Elements are been solved by expert teachers of CBSETuts.com. All the solutions given in this page are solved based on CBSE Syllabus and NCERT guidelines.
NCERT Exercises
Question 1.
Discuss the pattern of variation in oxidation states of
(i) B to TI and
(ii) C to Pb.
Solution.
The aspect common in the variation of oxidation state within group 13 (B to Tl) and group 14 (C to Pb) is that of inert pair effect. As we move down the group (13 or 14), we find that the lower oxidation state becomes more stable. In case of group 13 elements, it is +1 oxidation state that becomes stable while in group 14 it is +2 oxidation state that becomes stable. This behaviour can be understood if we consider the fact that as we move from the 2nd period to the 3rd a d-subshell is added. Similarly, upon moving further down the group there is an f-subshell which is added, f-subshells have a poor shielding effect. As a result, the s-electrons in the heavier elements of the 13th and 14th group are held more tightly and thus, are reluctant to get oxidised. This causes the lower oxidation state to become more stable.
|
Element |
Oxidation state |
Element |
Oxidation state |
|
B |
+3 |
C |
+4 |
|
Al |
+3 |
Si |
+4 |
|
Ga |
+1, +3 |
Ge |
+2, +4 |
|
In |
+1, +3 |
Sn |
+2, +4 |
|
TI |
+1, +3 |
Pb |
+2, +4 |
Question 2.
How can you explain higher stability of BCl3 as compared toTICl3?
Solution.
The higher stability of BCl3 as compared to TICl3 can be explained with the help of inert pair effect which becomes more stable as we move down the group. TI is more stable in its +1 oxidation state and hence TICl3 is not stable as TI shows an oxidation state of +3 in TICl3.
Question 3.
Why does boron trifluoride behave as a Lewis acid?
Solution.
B has an electronic configuration of 1s22s22p1 and undergoes sp2 hybridisation and then bonds with 3 fluorine atoms to yield BF3.

But, inspite of this bonding it remains electron deficient i.e., it does not have 8 electrons around it in the outermost shell; it has only 6 electrons in the outermost shell.
This electron deficiency coupled with the fact that it has an empty 2p orbital which can accept more electrons making it a Lewis acid. (Lewis acids are compounds that can accept electrons)
Question 4.
Consider the compounds, BCl3 and CCl4. How will they behave with water ? Justify.
Solution.
BCl3 is an electron deficient compound. Also, it has an empty unhybridised p-orbital which can accept electrons. In presence of water, BCl3 hydrolyses and forms B(OH)3.
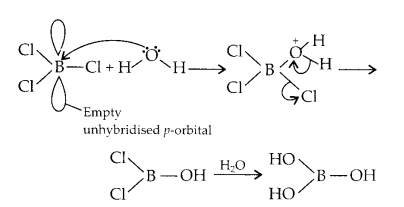
But, when CCl4 is mixed with water, no reaction takes place because carbon neither has any unhybridised, empty p-orbital where it can accommodate electrons from water nor it has empty d-orbital.
CCl4 + H2O ➝ No reaction
Question 5.
Is boric acid a protic acid? Explain.
Solution.
Boric acid – H3BO3 or B(OH)3, is not a protic acid (which are acids that donate protons). But an aqueous solution of boric acid is found to be weakly acidic in nature. This acidic character arises due to the Lewis acid character of boric acid which abstracts a hydroxyl ion from water and leaves free H+ ions which make the solution acidic.

Question 6.
Explain what happens when boric acid is heated.
Solution.
On heating orthoboric acid above 370 K, it forms metaboric acid, HBO2 which on further heating yields boric oxide, B2O3.
![]()
Question 7.
Describe the shapes of BF3 and B\({ H }_{ 4 }^{ – }\). Assign the hybridisation of boron in these species.
Solution.
BF3 has a planar triangular structure which arises from the sp2 hybrid orbitals.

These three sp² hybrid orbitals are directed towards the corners of triangle and BF³ has a trigonal structure.

B\({ H }_{ 4 }^{ – }\) may be assumed to be made of a central B atom, 3H atoms and one hydride ion H–.

In order to accommodate the 3H atoms and one H– ion, B undergoes sp3 hybridisation yielding four orbitals, 3 of which contain one e– each and one is empty. The fourth, empty orbital accomodates the H– ion. Thus, the structure of B\({ H }_{ 4 }^{ – }\) is tetrahedral.
Question 8.
Write reactions to justify amphoteric nature of aluminium.
Solution.
Amphoteric substances are those that can react with both acids and bases. Aluminium reacts with HCl to liberate H2 gas as :

Question 9.
What are electron deficient compounds? Are BCl3 and SiCl4 electron deficient species? Explain
Solution.

Question 10.
Write the resonance structures of \({ CO }_{ 3 }^{ 2- }\) and \({ HCO }_{ 3 }^{ – }\).
Solution.
The resonance structures of \({ CO }_{ 3 }^{ 2- }\) and \({ HCO }_{ 3 }^{ – }\) are :

Question 11.
What is the state of hybridisation of carbon in
(a) \({ CO }_{ 3 }^{ 2- }\)
(b) diamond
(c) graphite?
Solution.

Question 12.
Explain the difference in properties of diamond and graphite on the basis of their structures.
Solution.
|
Criterion |
Diamond |
Graphite |
|
Hybradisation |
Sp3 | Sp2 |
| Structure of C | Tetrahedral carbon which gives rise to a 3-dimensional structure. | Planar trigonal which gives rise to a 2-dimensional sheet like structure of carbon. |
| C – C | 154 pm | 141.5 pm |
| Hardness | Due to 3-D structure, diamond is the hardest natural element on the earth. | It is made up of 2-D sheets of carbon which slip over each other. This gives graphite a slippery surface. |
| Electrical Conductivity | Diamond is an insulator |
Graphite is a good conductor of electricity due to presence of delocalized π-electrons.
|
Question 13.
Rationalise the given statements and give chemical reactions:
(i) Lead (II) chloride reacts with Cl2 to give PbCI4.
(ii) Lead (IV) chloride is highly unstable towards heat.
(iii) Lead is known not to form an iodide, Pbl4.
Solution.

Question 14.
Suggest reasons why the B—F bond lengths in BF3 (130 pm) and \({ BF }_{ 4 }^{ – }\) (143 pm) differ.
Solution.
The bond length in anv compound is dependent on the hybridisation of the central atom. Boron in BF3 is sp2 hybridised which means that the s-character is 33% and therefore, the bond length is shorter. Also due to similar size of both atoms and vacant p-orbital of B, a pπ-pπ back bonding from F to B occurs causes partial double bond character. This further decreases the bond length of B — F. In \({ BF }_{ 4 }^{ – }\), the hybridisation of B is sp3 which means that the s-character is 25% and therefore, a longer bond length.
Question 15.
If B—Cl bond has a dipole moment, explain why BCl3 molecule has zero dipole moment.
Solution.
The dipole moment of any molecule is the vector sum total of each of the dipole moments. In BCl3, molecule, although the B-Cl bonds individually are polar, the resultant dipole moment becomes zero.

We can see that the dipole moments of B-1Cl and B-2Cl produce a resultant which is equal in magnitude but opposite in direction to B-3Cl and hence cancels it out. That is why the net dipole moment of BCl3, is zero.
Question 16.
Aluminium trifluoride is insoluble in anhydrous HF but dissolves on addition of NaF. Aluminium trifluoride precipitates out of the resulting solution when gaseous BF3 is bubbled through. Give reasons.
Solution.
AlF3 is insoluble in anhydrous HF but when little NaF is added the compound becomes soluble. On adding BF3 to the above solution, AlF3 is reprecipitated along with sodium tetrafluoroborate.

Question 17.
Suggest a reason as to why CO is poisonous.
Solution.
Carbon monoxide is a colourless, odourless gas which has a tendency to bind to haemoglobin (the oxygen carrying molecule in blood), forming a complex called carboxyhaemoglobin. This complex is 300 times more stable than oxyhaemoglobin complex and therefore, if once, formed can seriously hamper the oxvgen supply to different organs and ultimately can cause death. This is why CO is said to be so dangerous.
Question 18.
Flow is excessive content of CO2 responsible for global warming?
Solution.
CO2 molecule is transparent to UV radiation but is opaque to IR radiation. Due to this, it allows the UV radiations of sunlight to pass into earth’s atmosphere but does not allow heat, which is in the form of 1R radiation, to move out of earth’s atmosphere. This causes heating of the atmosphere and produces a greenhouse effect.
Question 19.
Explain structures of diborane and boric acid. Diborane:
Solution.

The four terminal hydrogen atoms and the two boron atoms lie in one plane. Above and below this plane, there are two bridging hvdrogen atoms. The four terminal B – H bonds are regular two centre-two electron bonds (2c – 2e) while the two bridge (B – H – B) bonds are different and can be described in terms of three centre-two electron bonds (3c-2e).

Each B atom uses sp3 hybrids for bonding. Out of the four sp3 hybrids on each B atom, one is without an electron shown in broken lines. The terminal B-H bonds are normal 2-centre-2-electron bonds but the two bridge bonds are 3-centre-2-electron bonds. The 3- centre-2-electron bridge bonds are also referred to as banana bonds.
Boric acid : In boric acid, planar B03 units are joined by hydrogen bonds to give a layered structure.

Question 20.
What happens when:
(a) Borax is heated strongly.
(b) Boric acid is added to water.
(c) Aluminium is treated with dilute NaOFI.
(d) BF3 is reacted with ammonia.
Solution.

Question 21.
Explain the following reactions :
(a) Silicon is heated with methyl chloride at high temperature in the presence of copper;
(b) Silicon dioxide is treated with hydrogen fluoride;
(c) CO is heated with ZnO;
(d) Hydrated alumina is treated with aqueous NaOH solution.
Solution.


Question 22.
Give reasons:
(i) Conc. HNO3 can be transported in aluminium container.
(ii) A mixture of dilute NaOH and aluminium pieces is used to open drain.
(iii) Graphite is used as lubricant.
(iv) Diamond is used as an abrasive.
(v) Aluminium alloys are used to make aircraft body.
(vi) Aluminium utensils should not be kept in water overnight.
(vii) Aluminium wire is used to make transmission cables.
Solution.

Question 23.
Explain why is there a phenomenal decrease in ionization enthalpy from carbon to silicon?
Solution.
Large decrease in ionisation potential from C to Si is due to increase in size of the atom and shielding effect.
Question 24.
How would you explain the lower atomic radius of Ga as compared to Al?
Solution.
This can be understood from the variation in the inner core of the electronic configuration. The presence of additional 10 d-electrons offer only poor screening effect for the outer electron from the increased nuclear charge in gallium. Consequently, the atomic radius of gallium (135 pm) is less than that of aluminium (143 pm).
Question 25.
What are allotropes? Sketch the structure of two allotropes of carbon namely diamond and graphite. What is the impact of structure on physical properties of two allotropes?
Solution.
The property due to which an element exists in two or more forms which differ in their physical and some of the chemical properties is known as allotropy and the various forms are called allotropes or allotropic modifications. Carbon exists in two allotropic forms crystalline and amorphous. The crystalline forms are diamond and graphite.

Diamond due to extended covalent bonding is the hardest natural substance on the earth.
with Al2(SO4)3 which is soluble in Water.
Question 26.

Solution.

Question 27.
In some of the reactions thallium resembles aluminium, whereas in others it resembles group I metals. Support this statement by giving some evidence.
Solution.

Question 28.

Solution.

Question 29.
What do you understand by
(a) inert pair effect
(b) allotropy and
(c) catenation?
Solution.
(a) Inert pair effect : The reluctance of ns2 pair in p-block elements having higher atomic number to take part in bond formation is called inert pair effect.
(b) Allotropy : The existence of an element in more than one form having different physical properties but same or slightly different chemical properties is called allotropy.
(c) Catenation : The property by virtue of which a large number of atoms of the same element get linked together through covalent bonds resulting in the formation of long chains, branched chains and rings of different sizes is called catenation.
Question 30.
A certain salt X, gives the following results :
(i) Its aqueous solution is alkaline to litmus.
(ii) It swells up to a glassy material Yon strong heating.
(iii) When conc. H2SO4 is added to a hot solution of X, white crystal of acid Z separates out.
Write equations for all the above reactions and identify X, Y and Z.
Solution.

Question 31.
Write balanced equations for:

Solution.

Question 32.
Give one method for industrial preparation and one for laboratory preparation of CO and CO2 each.
Solution.


Question 33.
An aqueous solution of borax is
(a) neutral
(b) amphoteric
(c) basic
(d) acidic.
Solution.

Question 34.
Boric acid is polymeric due to
(a) its acidic nature
(b) the presence of hydrogen bonds
(c) its monobasic nature
(d) its geometry.
Solution.
(b) the presence of hydrogen bonds
Question 35.
The type of hybridisation of boron in diborane is
(a) sp
(b) sp2
(c) sp3
(d) dsp2
Solution.
(c) sp3
Question 36.
Thermodynamically the most stable form of carbon is
(a) diamond
(b) graphite
(c) fullerenes
(d) coal
Solution.
(b) : Graphite is thermodynamically more stable than diamond and its free energy of formation is 1.9 kJ mol-1 less than diamond.
Question 37.
Elements of group 14
(a) exhibit oxidation state of +4 only
(b) exhibit oxidation state of +2 and +4
(c) form M2- and M4+ ions
(d) form M2+ and M4+ ions.
Solution.
(b, d): The group 14 elements have four electrons in outermost shell. The common oxidation states exhibited by these elements are +4 and +2.
Elements like Sn and Pb are metallic thus, form M2+ and M4+ ions.
Question 38.
If the starting material for the manufacture of silicones is RSiCl3, write the structure of the product formed.
Solution.

Now that you are provided all the necessary information regarding NCERT Solutions for Class 11 Chemistry Chapter 11 The pBlock Elements and we hope this detailed NCERT Solutions are helpful.
NCERT Solutions for Class 11 Chemistry
Class 11 Chemistry NCERT Solutions
- Some Basic Concepts of Chemistry Class 11 NCERT Solutions
- Structure of Atom Class 11 NCERT Solutions
- Classification of Elements and Periodicity in Properties Class 11 NCERT Solutions
- Chemical Bonding and Molecular Structure Class 11 NCERT Solutions
- States of Matter Class 11 NCERT Solutions
- Thermodynamics Class 11 NCERT Solutions
- Equilibrium Class 11 NCERT Solutions
- Redox Reactions Class 11 NCERT Solutions
- Hydrogen Class 11 NCERT Solutions
- The s Block Elements Class 11 NCERT Solutions
- The p Block Elements Class 11 NCERT Solutions
- Organic Chemistry: Some Basic Principles and Techniques Class 11 NCERT Solutions
- Hydrocarbons Class 11 NCERT Solutions
- Environmental Chemistry Class 11 NCERT Solutions