NEET Chemistry Chapter Wise Mock Test – Some Basic Principles and Techniques
Question 1:

Question 2:

Question 3:

Question 4:

Question 5:

Question 6:

Question 7:
Which of the following will have a meso – isomer also?
(a) 2-chlorobutane
(b) 2, 3-dichlorobutane
(c) 2, 3-dichloropentane
(d) 2-hydroxy propanoic acid
Question 8:
Number of optical isomers of lactic acid are
(a) 1
(b) 2
(c) 3
(d) 4
Question 9:
How many enantiomeric pairs are obtained by monochlorination of 2, 3-dimethyl butane?
(a) Four
(b) Two
(c) Three
(d) One
Question 10:
If there is no rotation of plane polarised light by a compound in a specific solvent, thought to be chiral, it may mean that
(a) the compound is certainly achiral
(b) the compound is certainly meso
(c) there is no compound rrt the solvent
(d) the compound maylSdirafcemic mixture
Question 11:
Among the following which one can have a meso form?
(a) CH3CH(OH)CH(CI)C2H5
(b) CH3CH(OH)CH(OH)CH3
(c) C2H5CH(OH)CH(OH)CH3
(d) HOCH2CH(CI)CH3
Question 12:

Question 13:

Question 14:
More stable carbocation is formed during heating of which one of the following compound with conc.H2SO4
(a) (CH3)3COH
(b) C6H5CH2OH
(c) (CH3)2CHOH
(d) CH3CH(OH)CH2CH3
Question 15:

Question 16:

Question 17:

Question 18:

Question 19:

Question 20:

Question 21:

Question 22:

Question 23:

Question 24:

Question 25:
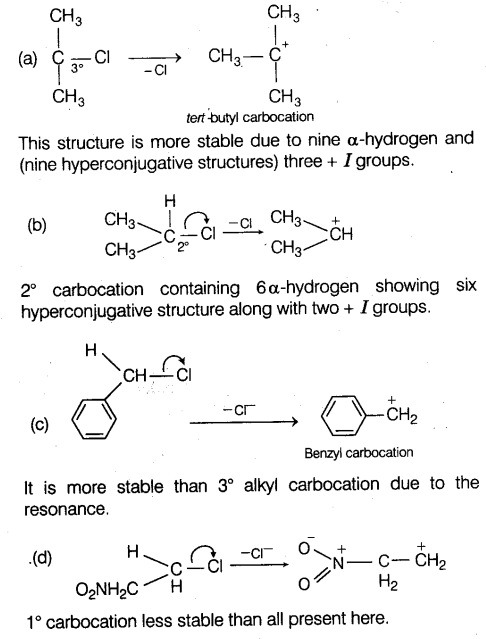
Hyperconjugation involves delocalisation
(a) electrons of carbon-hydrogen bond of an alkyl group directly linked to an atom of unsaturated system
(b) electrons of carbon-hydrogen bond of alkyl group directly attached to the negatively charged carbon atom
(c)π-electrons of carbon-carbon bond
(d) one pair of electrons
Question 26:

Question 27:

Question 28:
Dehydrohalogenation of an alkyl halide is
(a) nucleophilic substitution reaction
(b) elimination reaction
(c) rearrangement reaction
(d) Both (a) and (b)
Question 29:
Impure naphthalene is purified by
(a) fractional crystallisation
(b) Konal distillation
(c) solvent extraction
(d) sublimation
Question 30:
The best method for the separation of naphthalene and benzoic acid from their mixture is
(a) chromatography
(b) crystallisation
(c) distillation
(d) sublimation
Question 31:
Fractional distillation is useful in the distillation of
(a) petroleum
(b) coaltar
(c) crude alcohol
(d) All of these
Question 32:
Impure glycerine can be purified by
(a) steam distillation
(b) simple distillation
(c) vacuum distillation
(d) extraction with a solvent
Question 33:
Steam distillation is based on the fact that vaporisation of organic liquid takes place at
(a) lower temperature than its boiling point
(b) higher temperature than its boiling point
(c) its boiling point
(d) water and organic liquid both undergo distillation
Question 34:
The sodium extract of an organic compound on acidification with acetic acid and addition of lead acetate solution gives a black precipitate. The organic compound contains
(a) nitrogen
(b) halogen
(c) sulphur
(d) phosphorus
Question 35:
Copper wire test of halogens is known as
(a) Leibig’stest
(b) Lassaigne’s test
(c) Fusion test
(d) Beilstein’s test
Question 36:
During AgN03 test for detection of halogens, sodium extract is boiled with few drops of cone. HN03 to decompose
(a) NaCN
(b) Na2S
(c) Both (a) and (b)
(d) None of these
Question 37:

Question 38:
Sodium nitroprusside when added to an alkaline solution of sulphide ions, produce a
(a) red colouration
(b) blue colouration
(c) purple colouration
(d) brown colouration
Question 39:
0.5 g of hydrocarbon gave 0.9 g water on combustion. The percentage of carbon in hydrocarbon is
(a) 60.6
(b) 28.8
(c) 80.0
(d) 68.6
Question 40:
Percentage composition of an organic compound is as follows C = 10.06, H = 0.84, Cl = 89.10
Which of the following corresponds to its molecular formula if the vapour density is 60.0?
(a) CH3CI
(b) CHCI3
(c) CH2CI2
(d) None of these
Question 41:
Empirical formula of a compound is CH20 and its vapour density is 30. Molecular formula of the compound is
(a) C2H402
(b) C2H602
(C) C2H202
(d) C2H40
Question 42:
An organic compound contains about 52% carbon. It could be
(a) Only ethanol
(b) Only dimethyl ether
(c) Both (a) and (b)
(d) None of these
Direction (Q. Nos. 43-44) In the following questions more than one answers given may be correct. Select the correct answers and mark it according to the codes.
Codes
(a) 1,2 and 3 are correct
(b) 1 and 2 are correct
(c) 2 and 4 are correct
(d) 1 and 3 are correct
Question 43:
For resonance structure, a molecule should have
1. identical arrangement of atoms
2. nearly same energy content
3. the same number of paired electrons
4. identical bonding
Question 44:
Which of the following molecules contains(s) only primary hydrogen atoms?
(a) Neo-pentane
(b) Neo-hexane
(c) 2,2,3,3-tetramethylbutane
(d) 2,2,3-trimethylpentane
Question 45:

Question 46:

Direction (Q. NOS. 47-49) Each of these questions contains two statements : Assertion and Reason. Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
(a) Assertion is true, Reason is true; Reason is the correct explanation for Assertion
(b) Assertion is true, Reason is true; Reason is not the correct explanation for Assertion
(c) Assertion is true, Reason is false
(d) Assertion is false, Reason is true
Question 47:
Assertion In the test of.nitrogen, FeS04 is used.
Reason FeS04 cannot be easily oxidised.
Question 48:
Assertion A mixture of plant pigments can be separated by chromatography.
Reason Chromatography is used for the separation of coloured substances into individual components.
Question 49:
Assertion In the E2 elimination, p-H and leaving group should be antiperiplanar.
Reason In the E2 elimination, base always abstracts unhindered p-H.
Question 50:
In Duma’s method for estimation of nitrogen, 0.25 g of an organic compound gave 40 mL of nitrogen collected at 300 K temperature and 725 mm pressure. If the aqueous tension at 300 K is 25 mm, the percentage of nitrogen in the compound is
(a) 17.36
(b) 18.20
(c) 16.76
(d) 15.76
Question 51:

Question 52:

Question 53:

Question 54:


Question 55:
In the Kjeldahl’s for estimation of nitrogen present in a soil sample, ammonia evolved from 0.75 g of sample neutralised 10 mL of 1 M H2SO4 The percentage of nitrogen in the soil is
(a) 37.33
(b) 45.33
(c) 35.53
(d) 43,33
Question 56:
The number of isomer for the compound with the molecular formula C2BrCIFl is
(a) 3
(b) 4
(c) 5
(d) 6
Question 57:

Question 58:

Question 59:

Question 59:

Question 60:
Which of the following acids does not exhibit optical isomerism?
(a) Maleic acid
(b) a-amino acids
(c) Lactic acid
(d) Tartaric acid
Question 61:

Question 62:
Which one of the following undergoes nucleophilic substitution exclusively by SN1 mechanism?
(a) Benzyl chloride
(b) Ethyl chloride
(c) Chlorobenzene
(d) sopropyl chloride
Question 63:

Question 64:
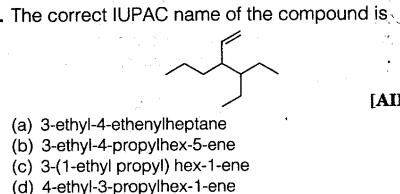
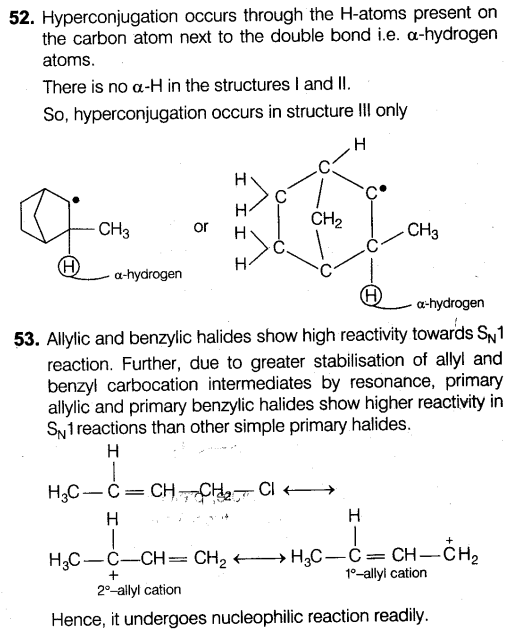
Question 65:

Question 66:
In Duma’s method of estimation of nitrogen, 0.35 g of an organic compound gave 55 mL of nitrogen collected at 300 K temperature and 715 mm pressure. The percentage composition of nitrogen in the compound would be
(Aqueous tension at 300 K = 15 mm)
(a) 16.45
(b) 17.45
(c) 14.45
(d) 15.45
Question 67:

Question 68:
Which one is the most reactive towards SN1 reaction?
(a) C6H5CH(C6H5)Br
(b) C6H5CH(CH3)Br
(c) C6H6C(CH3)(C6H5)Br
(d) C6H5CH2Br
Question 69:

Question 70:
In an organic compound, phosphorus is estimated as
(a) Mg2P207
(b) Mg3(P04)2
(c) H3P04
(d) P205
Question 71:

Question 72:

Answers:

Hints and Solutions