NEET Chemistry Chapter Wise Mock Test – Hydrocarbons
Question 1:
By Wurtz reaction, a mixture of methyl iodide and ethyl iodide gives
(a) butane
(b) ethane
(c) propane
(d) a mixture of the above three
Question 2:

Question 3:
Formation of alkane by the action of zinc on alkyl halide is called
(a) Wurtz reaction
(b) Kolbe’s reaction
(c) Ulmann’s reaction
(d) Frankland reaction
Question 4:
Which isomer of hexane has only two different sets of structurally equivalent hydrogen atoms?
(a) 2,2-dimethylbutane
(b) 2-methylpentane
(c) 3-methylpentane
(d) 2,3-dimethylbutane
Question 5:
The increasing order of reduction of alkyl halides with zinc and dilute HCI is
(a)R— C<R — I<R —Br
(b)R—Cl<R—Br<R — I
(c)R — I<R — Br<R—Cl
(d)R—Br<R — I<R—Cl
Question 6:
Ozonolysis of an organic compounds gives formaldehyde as one of the products. This confirms the presence of
(a) two ethylenic double bonds
(b) a vinyl group
(c) an iso-propyl group
(d) an acetylenic triple bond
Question 7:

Question 8:

Question 9:
A gas which reacts with aqueous KMn04 solution but does not give precipitates with ammoniacal Cu2CI2 solution is
(a) ethylene
(b) methane
(c) acetylene
(d) ethane
Question 10:
Addition of HI on double bond of propene yields isopropyl iodide as major product. It is because the addition proceeds through
(a) more stable carbocation
(b) more stable carboanion
(c) homolysis
(d) more stable free radical
Question 11:

Question 12:

Question 13:
Arrange the following hydrogen halides in the order of their decreasing reactivity with propene
(a) HCI > HBr > HI
(b) HBr > HI > HCI
(c) HI > HBr > HCI
(d) HCI > HI > HBr
Question 14:
Acid catalysed hydration of alkenes except ethene leads to the formation of
(a) primary alcohol
(b) secondary or tertiary alcohol
(c) mixture of primary and secondary alcohols
(d) mixture of secondary and tertiary alcohols
Question 15:

Question 16:
The best method to prepare cyclohexene from cyclohexanol is by using
(a) HBr
(b) cone. H3P04
(c) cone. HCI + ZnCI2
(d) cone. HCI
Question 17:
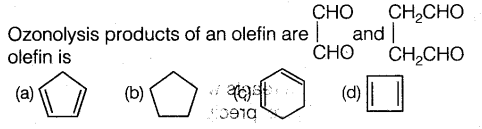
Question 18:

Question 19:
The enthalpy of combustion of H2, cyclohexene and cyclohexane are-241 ,-3800 and -3920 kJ per mol respectively. Heat of hydrogenation of cyclohexene is
(a) – 121 kJ per mol
(b) + 121 kJ per mol
(c) + 242 kJ per mol
(d) – 242 kJ per mol
Question 20:

Question 21:

Question 22:

Question 23:

Question 24:

Question 25:
Isopropyl benzene on air oxidation in the presence of dilute acid gives
(a) C6H5COOH
(b) C6H5COCH3
(c) C6H5CHO
(d) C6H5OH
Question 26:

Question 27:
The treatment of benzene with isobutene in the presence of sulphuric acid gives
(a) iso-butyl benzene
(b) n-butyl benzene
(c) fert-butyl benzene
(d) None of these
Question 28:
Toluene on oxidation with dil. HN03 gives
(a) benzaldehyde
(b) nitrotoluene
(c) benzoic acid
(d) phenol
Question 29:
Aromatisation of n-heptane by passing over (Al203 + Cr203) catalyst at 773 K gives
(a) benzene
(b) toluene ,
(c) mixture of both
(d) heptylene
Question 30:

Direction (Q. No. 31) In the following question more than one of the answers given may be correct. Select the correct answers and mark it according to the codes.
Codes
(a) 1,2 and 3 are correct
(b) 1 and 4 are correct
(c) 2 and 4 are correc
(d) 1 and 3 are correct
Question 31:
Which of the following can be used to distinguish between 1 -butyne and 2-butyne?
(a)Grignard reagent
(b) Ammoniacal AgN03
(c) Ammoniacal Cu2CI2
(d) Baeyer’s reagent
Question 32:

Question 33:
Assertion C—H bond in ethyene is shorter than C—H bond in ethene.
Reason Carbon atom in ethene is sp-hybridised while it is sp2 in ethyne.
Direction (Q. NOS. 33-34) Each of these questions contains two statements : Assertion and Reason. Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b) , (c) and (d) given below.
(a) Assertion is true, Reason is true; Reason is a correct explanation for Assertion
(b) Assertion is true, Reason is true; Reason is not a correct explanation for Assertion
(c) Assertion is true, Reason is false
(d) Assertion is false, Reason is true
Question 34:
Assertion Addition of HBr on 2-butene gives two isomeric products.
Reason Addition of HBr on 2-butene follows Markownikoff’s rule
Question 35:

Question 36:

Question 37:

Question 38:

Question 39:
Which of the following compounds will not undergo Friedel-Crafts reaction easily?
(a) Xylene
(b) Nitrobenzene
(c) Toluene
(d) Cumene
Question 40:

Question 41:
The function of AICI3 in Friedel-Crafts reaction is
(a) to absorb HCI
(b) to absorb water
(c) to produce nucleophile
(d) to produce electrophile
Question 42:

Question 43:

Question 44:

Question 45:
Which one of the following is aromatic?
(a) Cyclopentadienyl cation
(b) Cyclooctatetraene
(c) Cycloheptatriene
(d) Cycloheptatrienylcation
Question 46:
Angle strain in cyclopropane is
(a) 24°44′
(b) 9°44′
(c) 44′
(d)-5°16′
Question 47:

Question 48:
The reaction of toluene with Cl2 in the presence of FeCI3 gives ‘X’ and reaction in the presence of light gives Y’. Thus, ‘X’ and Y’ are [CBSE-AIPMT 2010]
(a) X = benzal chloride, Y = o-chlorotoluene
(b) X = n>chlorotoluene, Y = p-chlorotoluene
(c) X = o-and p-chlorotoluene,Y = trichloromethyl benzene
(d) X = benzyl chloride, Y = m-chlorotoluene
Question 49:

Question 50:

Question 51:
At room temperature, the eclipsed and staggered forms of ethane cannot be isolated because
(a) they interconvert rapidly
(b) both the conformers are equally stable
(c) the energy difference between the conformers is large
(d) there is a large energy barrier of rotation about the e-bond
Question 52:

Asnwers:

Hints and Solutions